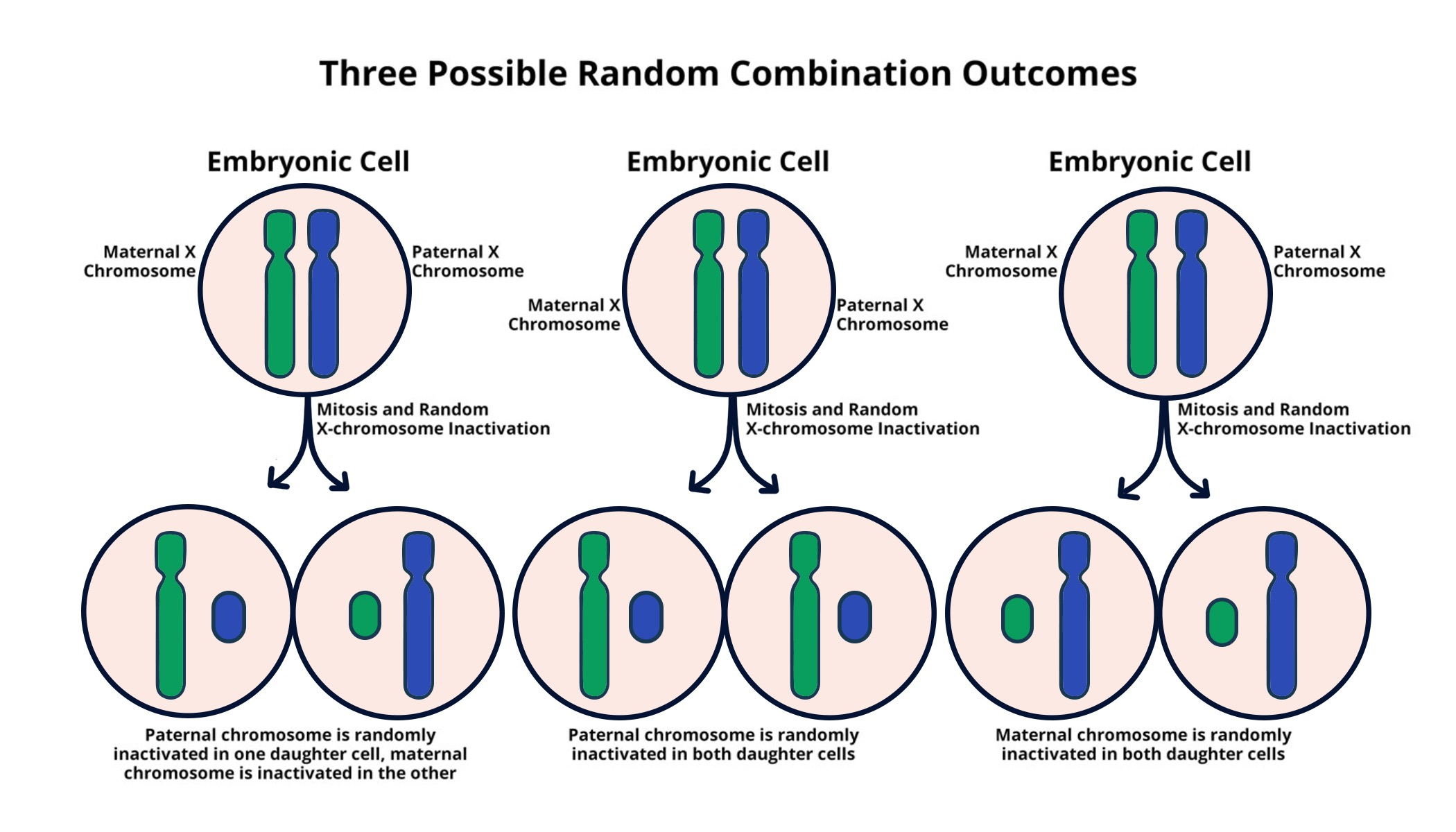
X chromosome inactivation is a fascinating process that has puzzled scientists for decades, particularly due to its critical role in gene regulation. In females, where two X chromosomes are present, one must be silenced to prevent an overload of gene expression. This chromosomal silencing is essential for the proper functioning of genes associated with various X-linked conditions, including Fragile X Syndrome and Rett Syndrome. Research led by Jeannie Lee has revealed intricate mechanisms behind this inactivation process, offering promising insights into potential gene therapy for X-linked diseases. By unraveling the complex interactions at play, her work opens avenues for innovative treatments that could significantly impact the lives of those affected by these genetic disorders.
The phenomenon of X chromosome inactivation, often referred to as XCI, serves a crucial purpose in balancing gene dosage between sexes. In females, where each cell carries two X chromosomes, the necessity to silence one is paramount to maintaining genetic equilibrium. This regulatory mechanism has vast implications for conditions like Fragile X and Rett syndromes, where mutations on the X chromosome lead to severe health issues. Jeannie Lee’s groundbreaking research continues to shed light on the dynamics of chromosomal silencing, paving the way for novel therapeutic approaches that may one day alleviate the symptoms of these genetic disorders. Understanding how XCI operates not only deepens our knowledge of genetics but also enhances the potential for developing effective treatments for X-linked diseases.
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a vital biological process in female mammals that ensures dosage compensation for X-linked genes. Females typically possess two X chromosomes, while males have only one, leading to a potential imbalance in gene expression. To maintain gene regulation, one of the X chromosomes in females is inactivated, which is crucial not only for normal development but also for the avoidance of gene dosage effects that could disrupt cellular functions. This intricate mechanism is orchestrated by various molecular players, including the Xist RNA, which initiates chromosomal silencing.
The discovery of how XCI operates has implications far beyond basic science. Understanding the dynamics of chromosomal silencing can unlock treatment avenues for X-linked diseases like Fragile X Syndrome and Rett Syndrome. Jeannie Lee’s groundbreaking research highlights the complexity of this process and its potential for therapeutic interventions. By freeing silenced genes using strategies developed in her lab, there’s hope to remedy conditions linked to mutated genes on the X chromosome, paving the way for innovative gene therapy for X-linked diseases.
Recent Advances in Genetic Therapies
Research advancements in genetic therapies are progressing rapidly, with particular emphasis on conditions like Fragile X and Rett syndromes. With a clearer understanding of XCI, scientists are now exploring gene therapy options that could target and potentially unsilence the affected X chromosome. This involves utilizing engineered molecules to disrupt the silencing process initiated by the Xist RNA, thus reestablishing the function of mutant genes crucial for normal neurological development.
Jeannie Lee’s team is taking significant strides toward bringing these therapies to clinical applications. Their recent studies indicate that by optimizing X-inactivation reversal techniques, it may be possible to treat individuals who carry mutations associated with these disorders. The combination of gene therapy approaches and precise targeting promises a new era in treating genetic conditions, which has been bolstered by substantial support from the National Institutes of Health.
Chromosomal Structure and Its Role in Disease
The physical structure of chromosomes plays a pivotal role in how genes are expressed and regulated. The gelatinous substance that coats chromosomes, likened to Jell-O, is crucial in maintaining the proper spatial organization required for effective gene expression. In diseases like Fragile X and Rett, alterations in chromosomal silencing contribute to the abnormal behavioral and developmental outcomes observed in affected individuals. Therefore, understanding this chromosomal architecture is paramount in developing effective treatment strategies.
Researchers like Jeannie Lee are dissecting the interactions between Xist RNA and the surrounding chromosomal material to unveil the mechanism behind gene silencing. Their findings indicate that by manipulating the Jell-O-like substance and its biophysical properties, scientists could restore functionality to previously inactive genes. The goal is to refine these interventions for application in clinical settings, ultimately offering hope for improved patient outcomes and a deeper understanding of chromosomal diseases.
Potential of Gene Therapy for X-Linked Conditions
Gene therapy represents a groundbreaking approach in treating inherited disorders, especially those linked to the X chromosome. Conditions like Fragile X Syndrome, characterized by cognitive impairments, can potentially be alleviated by unlocking the inactive, healthy allele throughout targeted gene therapies. This method promises a minimal side effect profile, primarily because it relies on the innate cellular mechanisms managing gene expression, in tandem with the existing, more stable genes.
Current research focuses on developing safe and efficient vectors for delivering therapeutic agents designed to modify the chromosomal behavior. The progress made by Lee’s lab showcases the possibility of reactivating silenced genes while sparing their healthy counterparts, a vital consideration in therapy development. This innovative approach could transform treatment paradigms for X-linked diseases, making previously untreatable conditions manageable.
The Future of Chromosomal Research
The future trajectory of chromosomal research hinges on unlocking the complexities of X chromosome inactivation and its implications for therapy. As the scientific community continues to delve deeper into the cellular and molecular mechanisms driving XCI, a host of therapeutic possibilities are emerging. Jeannie Lee’s pursuit not only illuminates the fundamental biology of this process but also heralds new eras of treatments for genetic disorders.
Investments in biotechnology and genetic research could enhance the speed at which new therapies are developed, pushing the boundaries of current medical treatments. With ongoing studies into the therapeutic potential of unsilencing X-linked genes, gene therapy may soon become a reality, offering hope to millions affected by these life-altering conditions. This ongoing quest underscores the intersection of scientific inquiry and clinical application, paving the way for groundbreaking treatments that could redefine patient care.
The Challenge of Silencing Mechanisms
Silencing mechanisms, while vital for regulating gene expression, pose challenges in developing targeted therapies for genetic disorders. The intricate dance between Xist and the surrounding chromatin has raised questions about selective gene targeting for therapeutic ends. For instance, understanding why certain genes remain silenced while others are activated is critical in leveraging these silencing mechanisms to our advantage during treatment development.
Navigating these challenges requires a multifaceted approach involving genetic, biochemical, and molecular biological techniques to elucidate the underlying principles governing gene silencing. As researchers like Jeannie Lee continue to explore these complexities, they enable the design of more precise interventions that could lead to breakthroughs in responsiveness to gene therapy, ultimately refining therapeutic strategies for those with conditions rooted in chromosomal abnormalities.
Clinical Implications of X Chromosome Research
The clinical implications of X chromosome research are profound, particularly in the context of X-linked genetic disorders. Treatments that target the reactivation of silenced genes offer a tantalizing glimpse into the future of personalized medicine. By harnessing the mechanisms of X chromosome inactivation, clinicians may soon provide targeted therapies that cater to the specific mutations underlying disorders such as Fragile X Syndrome and Rett Syndrome.
Moreover, the insights gained from this research extend beyond immediate treatment applications. They also illuminate potential preventative strategies, including early genetic screenings and interventions before symptoms manifest. Enhanced understanding fosters a proactive approach to genetic disorders, allowing for earlier diagnosis and potentially mitigating the impacts of these conditions before they significantly affect individuals’ lives.
Innovative Strategies in Neurodevelopmental Disorder Treatment
Innovative strategies are gaining traction in the field of neurodevelopmental disorders, particularly those associated with the X chromosome. With research focusing on unlocking the therapeutic potential of silenced genes, new methods are emerging that target the underlying pathophysiology of conditions like Fragile X and Rett syndromes. Lee’s lab is pioneering efforts to utilize chromosomal engineering to facilitate the reactivation of genes essential for normal brain development.
By employing advanced techniques in gene therapy, there is hope for reversing or alleviating the effects of genetic mutations associated with these disorders. The development of these innovative strategies can lead to tailored treatments that are more effective and safer for patients while minimizing side effects. As such, ongoing research in this area holds the key to transforming the landscape of disorder management and enhancing the quality of life for those affected.
Role of Chromatin in Gene Expression Regulation
Chromatin architecture plays a significant role in regulating gene expression and maintaining cellular functions. The dynamic structure of chromatin affects how genes are accessed for transcription, and understanding these interactions is crucial for developing therapies aimed at gene silencing. In the case of X chromosome inactivation, chromatin compactness allows for the selective silencing of one of the X chromosomes in females, a process integral to balanced gene expression.
Research such as that conducted by Jeannie Lee is uncovering how modifications to chromatin can facilitate or impede gene activation. As new techniques to modify the structure or interactions within chromatin emerge, the potential for reversing gene silencing becomes clearer. Such advancements could lead to novel therapeutic interventions for X-linked diseases, ultimately reshaping how clinicians approach treatment for such genetic disorders.
Frequently Asked Questions
What is X chromosome inactivation and why is it important?
X chromosome inactivation (XCI) is a cellular mechanism that randomly inactivates one of the two X chromosomes in female mammals. This process is crucial for dosage compensation, ensuring that females, with two X chromosomes, do not produce double the amount of X-linked gene products compared to males, who have one X chromosome. Understanding XCI is vital for addressing conditions linked to mutations on the X chromosome, including Fragile X Syndrome and Rett Syndrome.
How does X chromosome inactivation relate to Fragile X Syndrome treatment?
Fragile X Syndrome treatment research is closely linked to X chromosome inactivation. Studies focused on unsilencing the inactivated X chromosome can potentially allow the expression of healthy genes mutated in Fragile X Syndrome. Advances in understanding the chromosomal silencing mechanisms involved in XCI, such as the role of the RNA molecule Xist, could lead to innovative gene therapies that restore gene function in individuals with Fragile X Syndrome.
What role does Jeannie Lee’s research play in understanding X chromosome inactivation?
Jeannie Lee’s research at Harvard Medical School has significantly advanced our understanding of X chromosome inactivation (XCI). Her lab’s studies revealed the cellular mechanisms involved in chromosomal silencing and highlighted the critical role of the Xist RNA molecule in altering the properties of the chromosomal environment, which facilitates XCI. This research opens up possibilities for developing treatments for X-linked diseases like Fragile X Syndrome and Rett Syndrome.
Can gene therapy benefit from the knowledge gained about X chromosome inactivation?
Yes, gene therapy for X-linked diseases can greatly benefit from the insights gained about X chromosome inactivation. By understanding how to manipulate the inactivation process and reactivate the silent X chromosome, researchers can develop targeted therapies that may cure genetic disorders associated with mutations on the X chromosome. This approach has shown promise in early studies addressing conditions like Fragile X Syndrome and Rett Syndrome.
What are potential therapeutic strategies to reverse X chromosome inactivation?
Potential therapeutic strategies to reverse X chromosome inactivation involve using compounds that can target and unsilence the inactivated X chromosome. Research has shown that manipulating the chromosomal environment through agents that influence Xist or the properties of chromosomal silencing could allow for the expression of healthy genes in individuals with X-linked diseases, such as Fragile X Syndrome and Rett Syndrome.
How does chromosomal silencing relate to gene expression in X-linked diseases?
Chromosomal silencing is a key mechanism affecting gene expression in X-linked diseases. In females, one X chromosome is inactivated through X chromosome inactivation, which may hide healthy genes behind silenced chromosomes. Consequently, individuals with X-linked diseases may only express harmful mutations while healthy counterparts remain unavailable for cellular use. Research into XCI aims to uncover methods to reactivate these silent genes, providing potential treatments for disorders like Fragile X Syndrome.
What advancements have been made in treating Rett Syndrome through X chromosome inactivation research?
Advancements in treating Rett Syndrome are being explored through the framework of X chromosome inactivation research. The understanding of how XCI silences genes on the X chromosome has led to potential therapeutic strategies aimed at unsilencing mutated genes in Rett Syndrome. Researchers are working on developing compounds that could safely restore the function of genes, thus paving the way for effective treatments for this neurodevelopmental disorder.
| Key Aspect | Details |
|---|---|
| X Chromosome Inactivation | Occurs in females to prevent excess gene dosage since they have two copies of the X chromosome. |
| Role of Xist Gene | Instructs the production of an RNA molecule that alters the properties of surrounding chromosomal material. |
| Biophysical Changes | Xist changes the ‘Jell-O’ surrounding the chromosome to be more flexible, facilitating access to gene regions. |
| Therapeutic Implications | Potential to treat conditions like Fragile X Syndrome and Rett Syndrome by unsilencing healthy genes on the inactivated X chromosome. |
| Ongoing Research | Future studies aim to optimize treatments and conduct safety tests with hopes for clinical trials. |
| Unresolved Queries | Understanding why certain genes are unaffected when X-linked mutations are freed remains a mystery. |
Summary
X chromosome inactivation is a crucial biological process that allows female mammals to effectively manage their two X chromosomes by silencing one. This mechanism is essential to avoid duplicative gene products that could disrupt normal cellular function. Recent research, particularly from Jeannie T. Lee’s lab, has revealed exciting new insights into how Xist RNA and the surrounding chromosomal environment cooperate in this inactivation process. These findings not only enhance our understanding of genetic regulation but also open pathways for innovative treatments of diseases linked to the X chromosome, such as Fragile X and Rett Syndromes. Thus, ongoing studies and optimizations could potentially lead to revolutionary therapeutic solutions that target these X-linked genetic disorders.